My name is Krzysztof and I’m a new PhD student in the positronium spectroscopy group, but by no means new to UCL. I have recently completed my undergraduate degree in the Department of Chemistry and throughout my course I had multiple opportunities to work with various types of spectroscopy. In this short introduction I will present my recent experience in the Laboratory of Rotational Spectroscopy in the Institute of Physics of Polish Academy of Sciences (IF PAN).
Molecules, unlike unbound atoms, posses quantum states related to their relative nuclear motion. Nuclei within a molecule change their position with respect to one another while maintaining the centre of mass coordinate. One of these types of motions, vibration, can be described as if the nuclei were connected by an interatomic spring which oscillates at a certain frequency (Figure 1 a and c). Another type of motion, the main topic of this post, is rotation, where the molecule revolves around an axis and may be described by the classical motion of a spinning top or a merry-go-round (Figure 1 b and d).

In a classical picture, the energy of a rotor Erot(ω) is proportional to the moment of inertia I multiplied by the square of the angular velocity ω,
Erot(ω) = I ω2.
The angular velocity ω is defined as the change in orientation of a rotating body per unit of time (e.g. 1 rotation per second is 2π rad s-1), while the moment of inertia is a measure of the force needed to accelerate a rotating body.
On a molecular scale, the rotational energy is quantised, i.e. it can only take certain discrete values. For example, in the case of a linear molecule like carbon monoxide (Figure 1c), the rotational energy levels take values of integer multiples of hcB,
Erot(J) = h c B J ( J + 1 ),
where J is the rotational quantum number, B is the rotational constant, c is the speed of light in vacuum, and h is the Planck constant. The rotational quantum number is similar to the angular velocity ω in the classical case, while the constant B is proportional to moment of inertia of a molecule. This gives rise to discrete energy levels at 0, 2hcB, 6hcB, 12hcB…
This, and a very similar quantisation for vibrational energy, gives rise to a complex energy level structure on top of the electronic energy levels already present (see a previous article for an explanation of the electronic energy levels in atoms). Each electronic energy level possesses many vibrational states, usually denoted with vibrational quantum number ν. These states are not discussed in detail in this article, but shown in Figure 2 a. Each ν state has its own rotational structure with sub-states denoted with J, see Figure 2 b. It is worth noting that ν is used to represent the vibrational quantum number as well as for frequency.

It is possible to excite transitions between vibrational and rotational levels using photons, just as between electronic states. These transitions are subject to selection rules, and most predominantly occur in the infrared and microwave regimes of the electromagnetic spectrum. Spectroscopy in this region of the electromagnetic spectrum can be a perfect tool for astrophysical surveys of different molecular species in space, if coupled with a good theoretical foundation [2]. For more details about the principles of rotational spectroscopy refer to Atkins Physical Chemistry [3], or this short overview article from an experimental perspective [4].
Rotational spectroscopy is a great tool for studying the composition of the universe. By identifying transitions unique to a certain molecule, called fingerprint transitions, we can determine the molecular contents of the interstellar medium. Major collaborative experiments like the Atacama Large Millimeter/Submillimeter Array (ALMA), the Herschel Space Observatory for the Far-Infrared, the James Webb Space Telescope, and the recently launched Euclid mission (see the video from launch here!), collect data from vast regions of space. This data can be used to study the contents of the matter suspended between stars thanks to, among many techniques, rotational spectroscopy.
To identify a substance from the spectral data we need to have a very good model to reconstruct the molecular spectrum for a candidate species and compare it with actual data. To do that, we have to know certain characteristics of a molecule which we can obtain from a calculation or an experiment (preferably both).
The magnitude and orientation of the electric dipole moment μe is one of these fundamental properties which determine the molecular spectrum. The electric dipole moment of a molecule is defined as the separation between the point charges multiplied by their charge. As the intensity of the transition is proportional to , it is crucial to know the electric dipole moment in order to determine the relative population of the molecules in the environment being observed.
To probe μe we can use the Stark effect. In an electric field, the rotational transitions split into separate components, known as Stark lobes. The difference between the Stark shifted frequency νs and original frequency ν0, Δνs = νs – ν0, is approximately proportional to the square of the electric field. To read more about the Stark effect, refer to our previous article. Measurements of the Stark lobe frequency difference Δνs for n-propanol are shown in Figure 3.
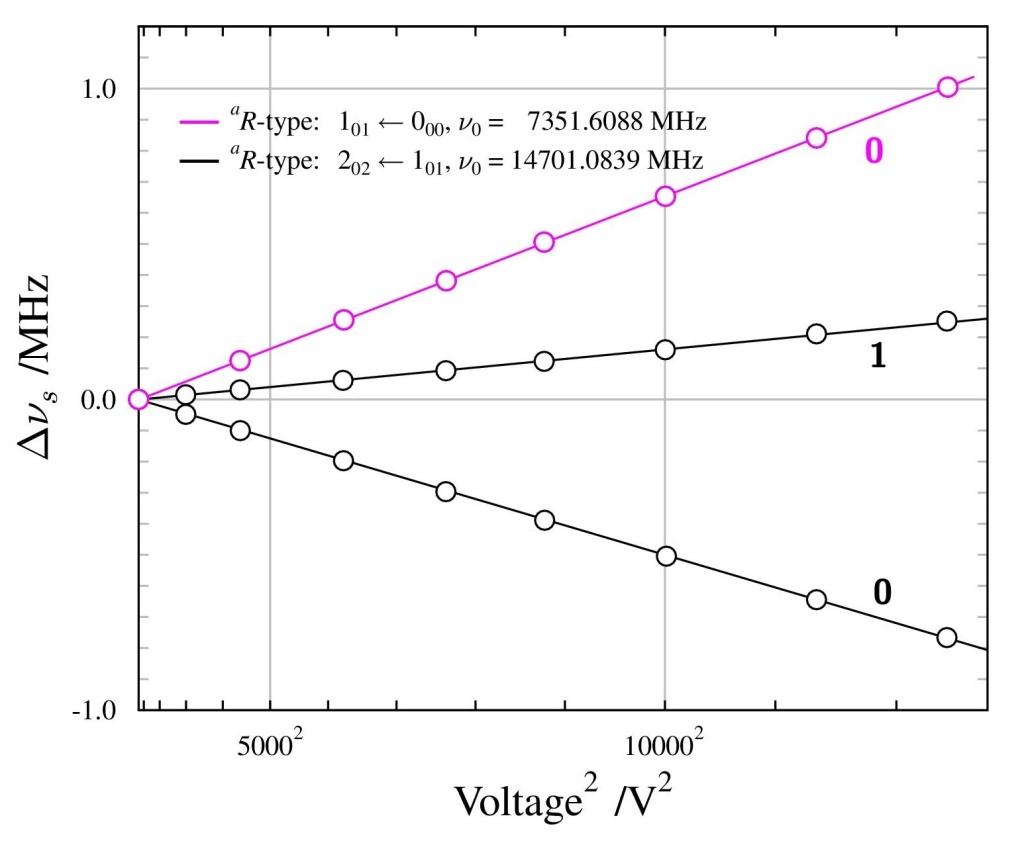
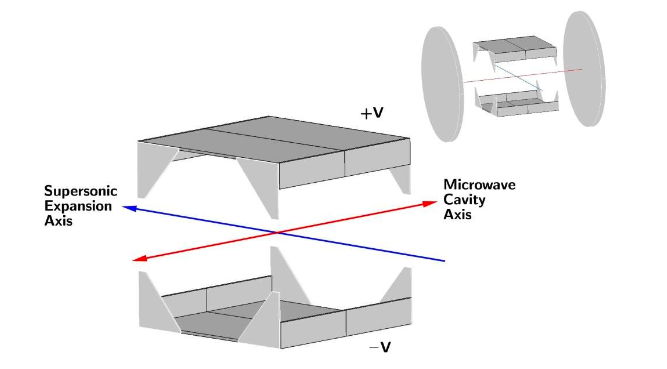
The transitions are measured by coinciding a low temperature supersonic beam of molecules with a microwave cavity, inside which is a region of well defined electric field. A schematic of this is shown in Figure 4. By measuring the energy splitting of the Stark lobes of transitions at different values of the electric field and fitting the obtained frequency shifts Δνs with a theoretical model [7] we can obtain the magnitude and orientation of the electric dipole moment, as already shown in Figure 3.
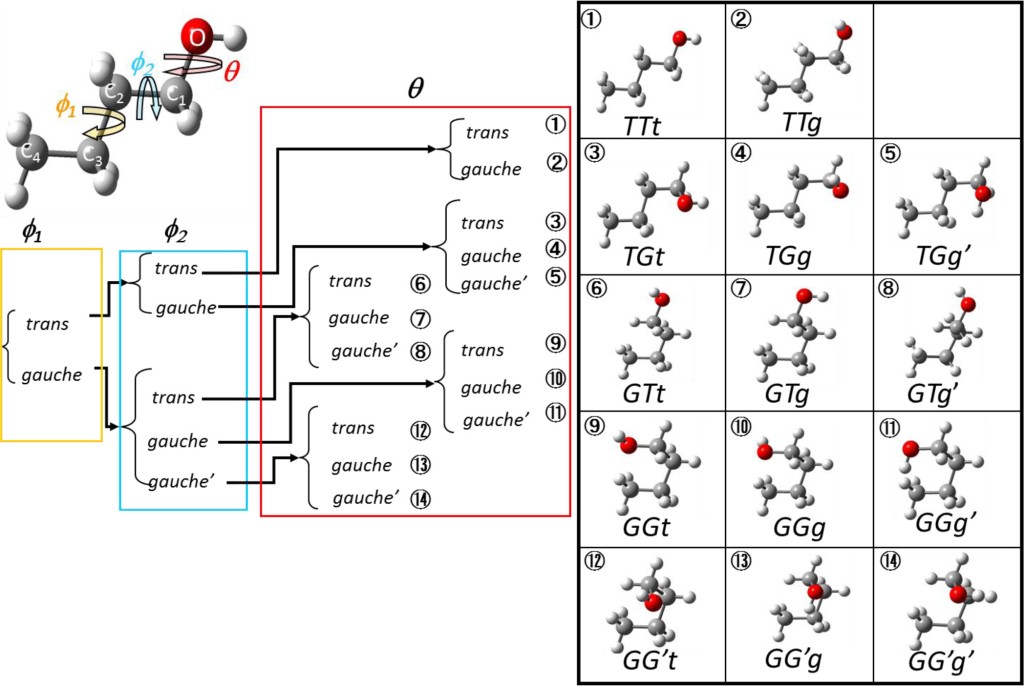
Ideally we would hope to record the spectrum of only one molecular species at a time to minimise any ambiguities. Unfortunately another complication, non existent in positronium or atomic spectroscopy, is freedom of the molecular bonds to rotate. The same molecule can occur in several different geometries, called conformations. For example, in n-butanol four bonds with torsional freedom result in 14 possible conformers, shown in Figure 5. Fortunately for the experiment, conformers vary in energy. Within the temperature regime of the molecules in this experiment, two conformers of n-butanol were fully characterised as the other, higher energy ones, were suppressed by the low temperature.
In summary, the electric dipole moment μe magnitude and orientation was experimentally determined for n-propanol using a Stark splitting measurement of rotational transitions. The values measured were a significant improvement in precision over previous measurements. For example, in n-propanol (Figure 6) the new experimental value of μe corresponds to an increase in intensity of some transitions by almost a factor of three with respect to previous results [5]. These findings aid astrophysical exploration of space by allowing more accurate analysis of spectral signals being acquired by multiple experiments.

Using positronium (the bound state of an electron and its antiparticle, the positron) you can measure the Rydberg constant with no perturbations from the less well understood quantum chromodynamics, which is present in each molecular species mentioned above and all conventional atoms with nuclei. This will require precise knowledge of the Stark effect and electric fields, as well as the use of millimetre-waves to measure electronic transition energies between high n Rydberg positronium states. This prior experience in experimental physics during my undergraduate studies was a great introduction to the kind of work which I will undertake with positronium during my doctorate.
[1] S. S. Harilal, B. E. Brumfield, N. L. LaHaye, K. C. Hartig, and M. C. Phillips, “Optical spectroscopy of laser-produced plasmas for standoff isotopic analysis”, Appl. Phys. Rev., vol. 5, 021301, 2018. DOI: 10.1063/1.5016053
[2] Z. Kisiel, “Assignment and Analysis of Complex Rotational Spectra” in Spectroscopy from Space. NATO Science Series, vol. 20. pp 91-106, Springer, Dordrecht (2000). DOI: 10.1007/978-94-010-0832-7_6
[3] P. Atkins, J. de Paula, and J. Keeler, “Topic 11B: Rotational Spectroscopy” in Atkins’ physical chemistry (12th ed.), pp 440-451, Oxford (2018). Access in BibliU
[4] Z. Kisiel, “THz Molecular Spectroscopy“, in Encyclopedia of Modern Optics (II ed.), pp 387-402 (2018), DOI: 10.1016/B978-0-12-803581-8.09638-7
[5] Z. Kisiel and K. Habdas, “Electric Dipole Moments from Stark Effect in Supersonic Expansion: n-Propanol, n-Butanol, and n-Butyl Cyanide“, Molecules, vol. 28, 1692, (2023) DOI: 10.3390/molecules28041692
[6] Z. Kisiel, J. Kosarzewski, and L. Pszczółkowski, “Nuclear Quadrupole Coupling Tensor of Ch2Cl2: Comparison of Quadrupolar and Structural Angles in Methylene Halides“, Acta Phys. Pol., vol. 92, 3, pp. 507-516, (1997) DOI: 10.12693/APhysPolA.92.507
[7] Y. Kawashima, Y. Tanaka, T. Uzuyama, and E. Hirota, “Conformations and Low-Frequency Intramolecular Motions of 1-Butanol, 1-Butanethiol, Iso-butanol, and Iso-butanethiol Investigated by Fourier Transform Microwave Spectroscopy Combined with Quantum Chemical Calculations“, J. Phys. Chem. A, vol. 125, 5, pp 1166-1183, (2021) DOI: 10.1021/acs.jpca.0c09687
[8] Z. Kisiel, J. Kosarzewski, B.A. Pietrewicz, L. Pszczółkowski, “Electric dipole moments of the cyclic trimers (H2O)2HCl and (H2O)2HBr from Stark effects in their rotational spectra“, Chem. Phys. Lett., vol. 325, 5-6, pp 523-530, (2000) DOI: 10.1016/S0009-2614(00)00729-6
Acknowledgements
Many thanks to Prof. Dr hab. Zbigniew Kisiel and ON2.3 laboratory of Vibrational and Rotational Spectroscopy for this great research experience. The Turing scheme travel fund is also appreciated.
